41 fda health claims on food labels
FDA to Redefine 'Healthy' Claim for Food Labeling Second, if a food labeled "healthy" meets the other requirements for the claim, FDA will exercise enforcement discretion if the only "beneficial" nutrient it contains at ten percent of the Daily Value (DV) or more is either potassium or vitamin D. These changes are consistent with the new nutrition labeling rules. FDA Food Label Requirements - Graphics Universal Incorporated Sugar content claims described in 21 CFR 101.60 (c), such as "sugar free" and "no sugar," are required to be accompanied by a statement that the food is "not a reduced calorie food," "not a low calorie food," or "not for weight control" if the food is not labeled as "low calorie" or "reduced calorie.".
Health Claims on Food Labels - LabelCalc Health claims, according to the FDA, are statements about the relationship between a food product or ingredient and a reduced risk of disease or a health condition. Basically, the FDA distinguishes two kinds of health claims: "authorized" and "qualified." Authorized Health Claims: Claims that have significant scientific agreement (SSA). This means there is a consensus in the publically available scientific information on the matter.

Fda health claims on food labels
CFR - Code of Federal Regulations Title 21 The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 101.1 - Principal display panel of package form food. § 101.2 - Information panel of package form food. § 101.3 - Identity labeling of food in packaged form. Claims on FDA-Regulated Food Product Labels | Registrar Typically, there are three categories of permitted claims: • Nutrient Content Claims. • Health Claims. • Structure/Function Claims. According to U.S. FDA, "Health Claims" are those that describe a relationship between a food, food component, or dietary supplement ingredient, and reducing risk of a disease or health-related condition. › food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Fda health claims on food labels. FDA perspectives on health claims for food labels | Request PDF Th e NLEA also gave the FDA authority to regulate health claims on food labels and in food labeling [3]. Under provisions of the NLEA, declarations for the content of total fat are to be expressed ... Introduction to Food Product Claims — FDA Reader A Qualified Health Claim is a statement approved by the FDA for use on food labels that has strict wording requirements. When there is emerging evidence between a food and the reduced risk of a disease or health condition, but not enough for the FDA to issue an Authorized Health Claim, the FDA may approve a "Qualified Health Claim". Reading Food Labels (for Parents) - Seattle Children's Hospital These claims must meet FDA standards. Some common food claims: Reduced fat or sugar means that a product has 25% less fat or sugar than the same regular brand. Light means that the product has 50% less fat or 1/3 less calories than the same regular product. Free means as little as possible of a nutrient, like sugar, fat, or gluten. Healthy ... › food › food-labeling-nutritionLabel Claims for Conventional Foods and Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
FDA perspectives on health claims for food labels - PubMed This law established mandatory nutrition labeling for most foods and placed restrictions on the use of food label claims characterizing the levels or health benefits of nutrients in foods. NLEA set a high threshold for the scientific standard under which the U.S. Food and Drug Administration (FDA) may authorize health claims, this standard is known as the significant scientific agreement (SSA) standard. Food Packaging Claims | American Heart Association It's important to understand what these claims mean so you can make informed decisions about the food you buy for yourself and your family. There are three categories of claims defined by statute and/or FDA regulations that can be used on food and dietary supplement labels: health claims, nutrient content claims, and structure/function claims. Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims, and structure/function claims. Learn more about these categories from Label Claims for Conventional Foods and Dietary Supplements. FDA: Labels Misleading on Major Food Brands March 3, 2010 - The FDA today warned 17 food makers -- including POM and Nestle -- that their "misleading" product labels violate federal law. The warning letters say the firms face having their ...
FDA Permits Qualified Health Claims on Nut Labels For the first time, the U.S. Food and Drug Administration has recognized a qualified health claim made by a conventional food. In a surprise move in July the FDA granted permission for peanuts, almonds, hazelnuts, pecans, pistachios and walnuts to carry a label touting their heart-healthy effects. "Scientific evidence suggests but does not ... CFR - Code of Federal Regulations Title 21 (1) health claim means any claim made on the label or in labeling of a food, including a dietary supplement, that expressly or by implication, including "third party" references, written statements... Food Labeling; Health Claim; Phytosterols and Risk of Coronary Heart ... Current § 101.83 now provides for a health claim on the label or labeling of a food meeting certain criteria provided the claim among other things: (1) States that plant sterol and plant stanol esters should be consumed as part of a diet low in saturated fat and cholesterol, (2) uses the term plant (or vegetable oil) sterol esters or plant (or vegetable oil) stanol esters, (3) specifies that the daily dietary intake necessary to reduce the risk of CHD is 1.3 g or more for plant sterol ... Food Labeling: An Introduction to Nutrient Content, Health, and Other ... Food Labeling: An Introduction to Nutrient Content, Health, and Other Claims Identify what is a claim, and who regulates claims on foods. Discuss the various types of claims. Understand the federal statutory authorities, related preemption, and state requirements. Riëtte van Laack , Director, Hyman, Phelps & McNamara, PC RIETTE VAN LAACK is a director
Questions and Answers on Health Claims in Food Labeling | FDA All health claims, whether authorized or qualified, require pre-market review by the FDA. Under federal law, the FDA approves by regulation authorized health claims for use in food labeling only if...
Health Claim Notification for Whole Grain Foods | FDA fda's decision not to prohibit or modify the claim means that as of july 8, 1999, the manufacturers may use the following claim on the label and in labeling of any product that meets the...
Food Labeling: Health Claims; Oats and Coronary Heart Disease The Food and Drug Administration (FDA) is announcing its decision to authorize the use, on food labels and in food labeling, of health claims on the association between soluble fiber from whole oats and a reduced risk of coronary heart disease (CHD). Based on its review of evidence submitted with...
FDA perspectives on health claims for food labels - ScienceDirect Volume 221, Issue 1, 3 April 2006, Pages 35-43 FDA perspectives on health claims for food labels ☆ J. CraigRowlands James E.Hoadley Get rights and content ☆ Presented in part at the Annual Meeting of American College of Nutrition, Long Beach, CA, 2004.
Food labelling and packaging: Nutrition, health claims and ... - GOV.UK Nutrition, health claims and supplement labelling Nutrition labelling You must follow nutrition labelling information rules for all pre-packed products unless both of the following apply: you're a...
Latest FDA developments on "Healthy" Claims on Food Labels Latest FDA developments on "Healthy" Claims on Food Labels $ 149.00 Recorded February 16, 2022 Session Summary FDA is moving full speed ahead on redefining the criteria for the nutrient content claim "healthy."
Health claim - Wikipedia A health claim on a food label and in food marketing is a claim by a manufacturer of food products that their food will reduce the risk of developing a disease or condition. For example, it is claimed by the manufacturers of oat cereals that oat bran can reduce cholesterol, which will lower the chances of developing serious heart conditions.
A Guide to FDA Regulation of Food Labeling Claims Among the FDA-regulated claims commonly declared on food labels are nutrient-content claims, health claims, qualified health claims and structure/function claims. Additionally, FDA has authority over claims related to gluten content, genetically modified organisms (GMOs) and "natural."
What are some examples of an FDA health claim on a food label? Correspondingly, what health claims are allowed on food labels? Approved Health Claims Calcium, Vitamin D, and Osteoporosis. Dietary Lipids (Fat) and Cancer. Dietary Saturated Fat and Cholesterol and Risk of Coronary Heart Disease. Dietary Non-cariogenic Carbohydrate Sweeteners and Dental Caries.
Food Politics by Marion Nestle » FDA study: Do added nutrients sell products? (Of course they do)
5 Understanding Food Labels and Health Claims - Maricopa Health Claims & Foods To keep companies from making false claims, the FDA provides food manufacturers' regulations in putting labels on packages that promote health. There are three levels of health claims: A health claim is supported by scientific evidence. An example is "reduces heart disease."
FDA Is Redefining The Term 'Healthy' On Food Labels | KGOU Now the FDA has begun the process of redefining the term "healthy" on food labels. Policymakers are looking for input from food makers, health experts and the public. You can weigh in with your ideas about what factors and criteria should be used for the new definition. ( Submit electronic comments directly to the FDA).
Food labeling: health claims; soy protein and coronary heart disease ... The Food and Drug Administration (FDA) is authorizing the use, on food labels and in food labeling, of health claims on the association between soy protein and reduced risk of coronary heart disease (CHD). Based on its review of evidence submitted with comments to the proposed rule, as well as evide …
FDA to Redefine "Healthy" Claim for Food Labeling FDA today announced that it has started a public process to redefine the "healthy" nutrient content claim for food labeling.
en.wikipedia.org › wiki › Food_and_Drug_AdministrationFood and Drug Administration - Wikipedia The United States Food and Drug Administration (FDA or USFDA) is a federal agency of the Department of Health and Human Services.The FDA is responsible for protecting and promoting public health through the control and supervision of food safety, tobacco products, dietary supplements, prescription and over-the-counter pharmaceutical drugs (medications), vaccines, biopharmaceuticals, blood ...
› food › food-labeling-nutritionLabel Claims for Food & Dietary Supplements | FDA Mar 07, 2022 · Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims ...
Claims on FDA-Regulated Food Product Labels | Registrar Typically, there are three categories of permitted claims: • Nutrient Content Claims. • Health Claims. • Structure/Function Claims. According to U.S. FDA, "Health Claims" are those that describe a relationship between a food, food component, or dietary supplement ingredient, and reducing risk of a disease or health-related condition.
CFR - Code of Federal Regulations Title 21 The information on this page is current as of Jan 06, 2022. For the most up-to-date version of CFR Title 21, go to the Electronic Code of Federal Regulations (eCFR). § 101.1 - Principal display panel of package form food. § 101.2 - Information panel of package form food. § 101.3 - Identity labeling of food in packaged form.




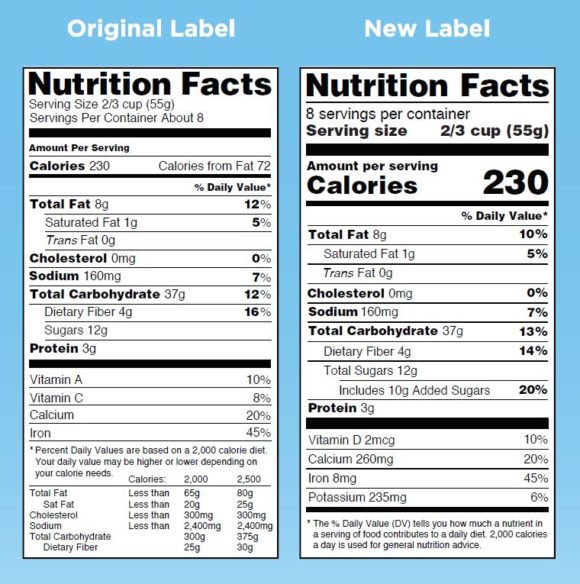







Post a Comment for "41 fda health claims on food labels"